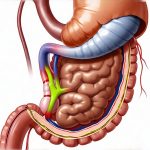
Diagnosing rare digestive disorders presents unique challenges. Unlike common conditions with well-defined diagnostic pathways, these illnesses often masquerade as more frequent ailments, leading to significant delays in accurate identification. Patients may endure years of misdiagnosis and inappropriate treatments before finally receiving a correct diagnosis, impacting their quality of life substantially. The scarcity of information about these conditions, coupled with limited awareness among healthcare professionals, further complicates the process. Often, the diagnostic journey requires innovative thinking and a departure from standard protocols to truly understand what is happening within the patient’s digestive system.
The difficulty isn’t just about recognizing unusual symptoms; it’s also about accessing appropriate testing. Many conventional tests are simply not sensitive enough to detect subtle abnormalities characteristic of rare disorders. Furthermore, specialized tests may only be available at a handful of centers globally, requiring patients to travel extensively or rely on telemedicine consultations with experts. The lack of established biomarkers and reliable diagnostic criteria often necessitates a holistic approach that integrates clinical observation, detailed patient history, cutting-edge research, and a willingness to explore unconventional testing strategies. This article will delve into some of these unusual approaches, highlighting how clinicians are striving to improve the diagnosis of these complex conditions.
Novel Biomarker Discovery & Metabolomics
Traditional diagnostic tests often focus on identifying known markers of disease. However, in rare digestive disorders, these markers may be absent or unreliable. Metabolomics, the large-scale study of small molecules (metabolites) within an organism, offers a promising alternative. By analyzing metabolites present in blood, urine, stool, or tissue samples, researchers can identify unique metabolic signatures associated with specific diseases – even those previously unknown. This approach moves beyond looking for what should be there to discovering what is different, potentially revealing early indicators of disease and personalized diagnostic tools.
The power of metabolomics lies in its ability to detect subtle changes that conventional tests might miss. For example, in certain rare metabolic disorders affecting digestion, specific metabolites accumulate due to enzymatic deficiencies. These accumulations can serve as “fingerprints” for the condition. Similarly, altered gut microbial metabolism – a key aspect of digestive health – can produce unique metabolite profiles indicative of underlying dysfunctions. Applying advanced analytical techniques like mass spectrometry and nuclear magnetic resonance spectroscopy allows scientists to identify and quantify these metabolites with high precision.
This field is still evolving, but significant progress is being made in identifying biomarkers for rare conditions like mitochondrial disorders affecting gut motility or specific forms of intestinal failure. The challenge lies in validating these biomarkers across larger patient cohorts and translating them into clinically useful diagnostic tests. Beyond biomarker identification, metabolomics can also help understand disease mechanisms and predict treatment responses, paving the way for more targeted therapies. Consider how optimizing best hydration strategies can support overall metabolic function.
Stool-Based Diagnostics: Beyond Traditional Analysis
Stool analysis is a cornerstone of digestive health assessment, but traditional methods often fall short in diagnosing rare disorders. Next-generation sequencing (NGS) applied to stool samples – known as 16S rRNA gene sequencing or metagenomic sequencing – provides an unprecedented level of detail about the gut microbiome. This can reveal imbalances in bacterial composition (dysbiosis) associated with specific conditions, even those not readily apparent through conventional culture methods. Identifying the presence of unusual microbial species or detecting alterations in microbial metabolic pathways can offer valuable diagnostic clues.
Beyond identifying who is present, advanced stool analysis can also assess what microbes are doing. Metagenomic sequencing allows researchers to analyze the collective genetic material of all microorganisms in a sample, revealing their functional capabilities. This provides insights into how the microbiome contributes to digestion, nutrient absorption, and immune regulation. For example, in rare disorders affecting bile acid metabolism, stool analysis can identify alterations in microbial bile salt hydrolase activity – an enzyme crucial for processing bile acids.
Furthermore, innovative techniques are emerging that analyze fecal calprotectin levels with higher sensitivity or detect specific markers of intestinal inflammation not captured by standard assays. This is particularly valuable in diagnosing conditions like microscopic colitis or eosinophilic gastroenteritis, which can present with subtle symptoms and require specialized testing. Combining NGS with metabolomic analysis of stool samples offers a powerful synergistic approach to unraveling the complex interplay between gut microbes, metabolites, and disease pathogenesis. Understanding these microbial interactions is crucial, especially when considering meal planning for sensitive systems.
Breath Tests: Detecting Subtle Gas Production Anomalies
Breath tests are widely used in diagnosing conditions like lactose intolerance or small intestinal bacterial overgrowth (SIBO). However, their application can be expanded to detect subtle gas production anomalies indicative of rare digestive disorders. Hydrogen breath testing, traditionally used for carbohydrate malabsorption, can be modified to assess the metabolism of unusual sugars or fiber types associated with specific metabolic deficiencies. For instance, in certain rare genetic conditions affecting sugar digestion, patients may exhibit abnormal hydrogen production after consuming a unique disaccharide.
More sophisticated breath tests utilize stable isotope ratios to assess gut transit time and microbial fermentation patterns. Acetylene breath testing can identify impaired carbohydrate metabolism in the small intestine, potentially indicating underlying enzymatic deficiencies or bacterial overgrowth. Furthermore, analyzing other gases beyond hydrogen and methane – such as carbon dioxide or sulfide – can provide additional diagnostic information. The challenge lies in establishing normal ranges for these gases and differentiating between physiological variations and pathological findings.
The development of portable breath testing devices with enhanced sensitivity and specificity is further expanding the utility of this non-invasive diagnostic tool. These devices allow for real-time monitoring of gas production, providing valuable insights into digestive function and microbial activity. Integrating breath test data with other clinical information – such as symptom patterns, dietary habits, and genetic predispositions – can significantly improve diagnostic accuracy. Dietary adjustments are often necessary; exploring daily eating maps can be incredibly helpful for managing these conditions.
Capsule Endoscopy & Advanced Imaging Techniques
While conventional endoscopy is a mainstay in diagnosing many digestive disorders, it has limitations when exploring the small intestine—a common site of pathology in rare conditions. Capsule endoscopy involves swallowing a tiny wireless camera that travels through the entire digestive tract, capturing images along the way. This allows for visualization of areas inaccessible to traditional endoscopes, such as the jejunum and ileum. While helpful, interpreting capsule endoscopy images can be challenging, requiring specialized expertise.
Advanced imaging techniques are pushing the boundaries of diagnostic capabilities. Double-balloon enteroscopy allows for deeper access into the small intestine, enabling biopsies and therapeutic interventions. Furthermore, advancements in computed tomography (CT) and magnetic resonance imaging (MRI) – including diffusion-weighted imaging and functional MRI – can provide detailed information about intestinal inflammation, motility, and vascularity. These techniques are particularly valuable in diagnosing conditions like Crohn’s disease or vasculitis affecting the digestive tract.
Artificial intelligence (AI) is emerging as a powerful tool for analyzing medical images. AI algorithms can be trained to identify subtle patterns indicative of disease, improving diagnostic accuracy and reducing inter-observer variability. For example, AI can assist in detecting minute ulcerations or areas of inflammation on capsule endoscopy images, aiding clinicians in making more informed decisions. The integration of AI with advanced imaging techniques holds tremendous promise for enhancing the diagnosis of rare digestive disorders. Utilizing enzyme supplements may also support improved digestion and nutrient absorption, complementing these diagnostic methods. A focus on cooked versus raw food strategies can further aid in symptom management. Finally, exploring filling grain bowls provides a digestible and nutritious meal option.
The diagnostic journey for rare digestive disorders is often arduous and complex. However, by embracing novel testing strategies, leveraging advancements in technology, and fostering collaboration between clinicians and researchers, we can improve our ability to accurately identify these conditions and provide patients with the care they deserve. The ongoing development of biomarker discovery programs, advanced stool analysis techniques, innovative breath tests, and cutting-edge imaging technologies offers hope for a future where early diagnosis and personalized treatment are within reach for all individuals affected by these challenging illnesses.