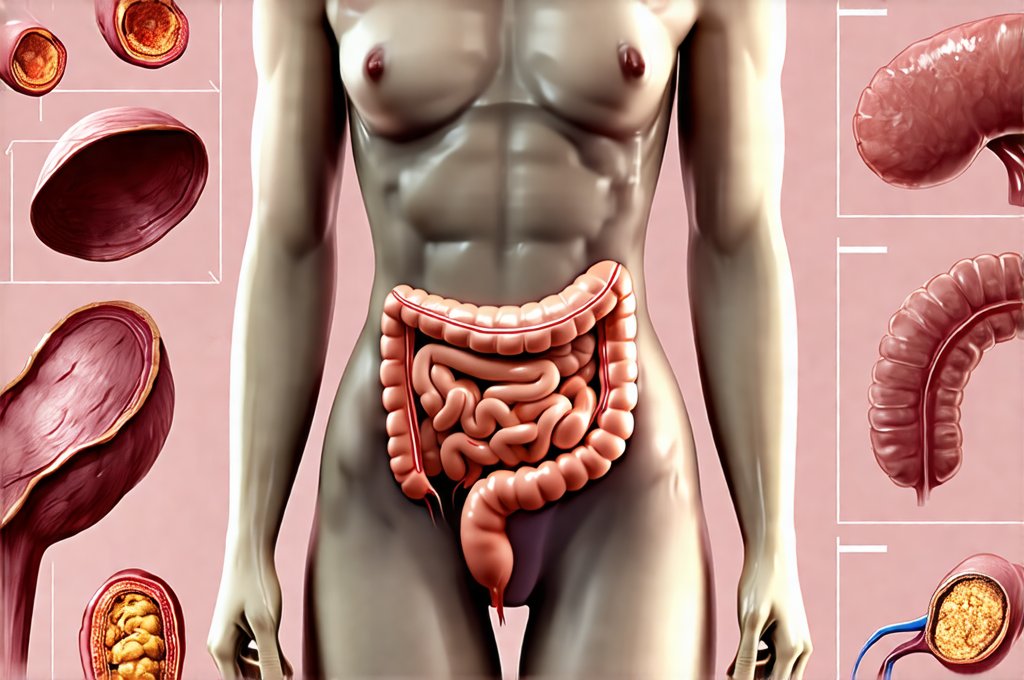
The human body is an intricate ecosystem, a complex interplay between genetics, lifestyle, and environment. For decades, diagnostic approaches have largely focused on isolating individual systems – cardiology assessing the heart, dermatology examining the skin, gastroenterology focusing on the digestive tract. However, increasingly sophisticated research reveals a profound interconnectedness, suggesting that imbalances in one area can ripple throughout the entire body, manifesting as seemingly unrelated symptoms. This holistic perspective is driving a shift towards more comprehensive assessments, moving beyond siloed diagnostics to embrace “full-body panels” designed not just to identify disease but to understand the underlying root causes of ill health. These advanced panels are no longer simply testing for what’s wrong; they’re seeking clues about why something went wrong, and increasingly, those clues are pointing directly towards the gut microbiome.
Traditional medical assessments often react to symptoms once they become pronounced. Full-body panels aim to be proactive, identifying potential vulnerabilities or imbalances before they escalate into full-blown disease. These aren’t replacements for standard clinical evaluations but rather complementary tools that provide a broader and more nuanced understanding of an individual’s health status. The most advanced iterations are incorporating markers related to gut health – microbial diversity, intestinal permeability (“leaky gut”), short-chain fatty acid production, and inflammatory responses – recognizing the central role the gut plays in systemic wellbeing. This isn’t merely about digestive issues; it’s about understanding how a compromised gut can influence everything from mental health and immune function to hormonal balance and chronic disease risk. The goal is personalized intervention based on a detailed map of individual physiology. Considering meal timelines can be an important step in this process.
Comprehensive Biomarker Analysis: Beyond Traditional Testing
The evolution of full-body panels has been driven by advancements in analytical technologies, particularly in genomics, metabolomics, and proteomics. Early panels often focused on basic blood chemistry (complete blood count, metabolic panel) and perhaps vitamin/mineral levels. Modern iterations go far beyond this, incorporating a wider range of biomarkers that offer deeper insights into physiological function. – Genomic testing can identify genetic predispositions to certain conditions, including those related to gut health (e.g., lactose intolerance, celiac disease). – Metabolomics analyzes the small molecules produced during metabolism, providing information about biochemical pathways and identifying imbalances indicative of gut dysbiosis or inflammation. – Proteomics examines the proteins present in blood or other bodily fluids, revealing markers of immune activation, tissue damage, or metabolic stress. This allows for a much more granular understanding of what is happening within the body, extending beyond simple diagnostic labels to reveal functional impairments. Focusing on hydration-timed meals can support this process.
The inclusion of gut-related biomarkers is truly transformative. Traditionally, assessing gut health meant relying on symptom reports (bloating, constipation, diarrhea) and sometimes stool tests that offered limited information. Now, panels can directly assess: – Microbial diversity: Measuring the richness and variety of microbial species in the gut. Lower diversity often indicates a less resilient ecosystem. – Intestinal permeability (“leaky gut”): Identifying markers like zonulin, which indicate increased intestinal permeability allowing undigested food particles and toxins to enter the bloodstream. This can trigger systemic inflammation. – Short-chain fatty acid (SCFA) production: Measuring levels of butyrate, propionate, and acetate – metabolites produced by gut bacteria that have profound effects on health. – Gut inflammation markers: Assessing inflammatory cytokines and antibodies related to food sensitivities or autoimmune responses triggered by the gut. This level of detail allows for targeted interventions aimed at restoring gut function and improving overall health. A strategy involving prep-ahead meals can be especially helpful here.
The power lies in the interconnectedness. It’s not enough to know you have low vitamin D; it’s crucial to understand why. Is it due to inadequate dietary intake, poor absorption (potentially linked to gut dysbiosis), or insufficient sunlight exposure? Full-body panels, with their emphasis on gut health, can help unravel these complexities and tailor interventions accordingly. This approach moves away from a “one-size-fits-all” mentality toward precision wellness strategies.
The Gut-Brain Connection & Mental Wellbeing
The link between the gut microbiome and brain function – often referred to as the gut-brain axis – is one of the most exciting areas of current research. The gut isn’t just a digestive organ; it’s a major communication hub, influencing mood, cognition, and even behavior. This bidirectional relationship operates through several pathways: – The vagus nerve, which directly connects the gut to the brain. – Production of neurotransmitters like serotonin (the “happy hormone”), much of which is produced in the gut. – Immune system modulation; gut health significantly influences immune function, impacting inflammation levels that can affect brain health. – Microbial metabolites reaching the brain and influencing neuronal activity.
Dysbiosis – an imbalance in the gut microbiome – has been linked to a range of mental health conditions, including anxiety, depression, and even neurodegenerative diseases. Full-body panels incorporating gut biomarkers can help identify potential imbalances that may be contributing to these issues. For example, low levels of butyrate (an SCFA produced by beneficial bacteria) have been associated with increased inflammation and mood disorders. Similarly, an overgrowth of certain bacterial species can produce metabolites that negatively impact brain function. Identifying these imbalances allows for targeted interventions such as dietary changes, probiotic supplementation, or stress management techniques aimed at restoring gut health and improving mental wellbeing. Consider incorporating herb-infused foods to support this process.
Restoring a healthy gut microbiome isn’t just about physical health; it’s about nurturing the foundation for optimal cognitive function and emotional resilience. The growing body of evidence highlights the importance of considering the gut when addressing mental health concerns – a paradigm shift from traditional approaches that have largely focused on pharmaceutical interventions alone. Personalized nutrition plans designed to support a thriving microbiome, alongside stress reduction strategies, can be powerful tools in promoting long-term mental wellness.
Immune Function & Gut Health: A Symbiotic Relationship
Approximately 70-80% of the immune system resides within the gut. The intestinal barrier acts as a critical line of defense, preventing harmful pathogens and toxins from entering the bloodstream while allowing for selective absorption of nutrients. The gut microbiome plays a vital role in “training” the immune system, helping it distinguish between friend and foe. Beneficial bacteria compete with pathogenic microbes, produce antimicrobial substances, and stimulate the production of antibodies. A healthy gut microbiome is essential for maintaining a balanced and responsive immune system.
When the intestinal barrier becomes compromised – often referred to as “leaky gut” – undigested food particles, toxins, and pathogens can enter the bloodstream, triggering an inflammatory response. This chronic inflammation can contribute to autoimmune diseases, allergies, and other health problems. Full-body panels that assess intestinal permeability markers (like zonulin) alongside immune function biomarkers (like immunoglobulin levels and cytokine profiles) can provide valuable insights into this intricate relationship. – Elevated zonulin suggests increased intestinal permeability, potentially leading to systemic inflammation. – Abnormal cytokine levels indicate an imbalanced immune response, possibly triggered by gut dysbiosis or leaky gut.
A key aspect of immune support is nourishing the microbiome. Dietary interventions focusing on fiber-rich foods, fermented foods, and prebiotics can promote the growth of beneficial bacteria and strengthen the intestinal barrier. Supplementation with probiotics (live microorganisms) may also be helpful in restoring microbial balance, but it’s crucial to choose strains that are appropriate for individual needs. The goal is not simply to boost the immune system; it’s to modulate it – ensuring a balanced and responsive immune response capable of effectively defending against threats without overreacting. Slow-eating meal routines can also support this modulation.
Personalized Interventions: Tailoring Treatment Based on Panel Results
The true value of full-body panels lies in their ability to inform personalized interventions. Generic recommendations rarely work for everyone; individuals respond differently to dietary changes, supplements, and lifestyle modifications based on their unique genetic makeup, gut microbiome composition, and physiological state. The detailed data provided by these panels allows healthcare practitioners to tailor treatment plans that are specifically designed to address each individual’s needs.
Here’s a hypothetical example: 1. A panel reveals low microbial diversity and elevated intestinal permeability in an individual experiencing chronic fatigue and brain fog. 2. Further investigation identifies specific bacterial deficiencies and food sensitivities based on the panel results. 3. An intervention plan is developed that includes: – Dietary changes to eliminate trigger foods and increase fiber intake. – Probiotic supplementation with strains known to promote gut diversity and barrier function. – Stress management techniques to reduce cortisol levels, which can exacerbate intestinal permeability. – Targeted nutrient support to address any identified deficiencies. The ongoing monitoring of biomarkers through follow-up panels allows for adjustments to the intervention plan as needed, ensuring optimal results. Liquid meal strategies can also be incorporated into this plan.
Personalization is not just about choosing the right supplement; it’s about creating a holistic health strategy that addresses the root causes of illness. It requires a collaborative approach between healthcare practitioners and patients, utilizing data-driven insights to empower individuals to take control of their health. The future of medicine is moving towards this level of precision – recognizing that each individual is unique and deserves a tailored approach to wellness. One-dish meals can be a great starting point for personalized nutrition plans.