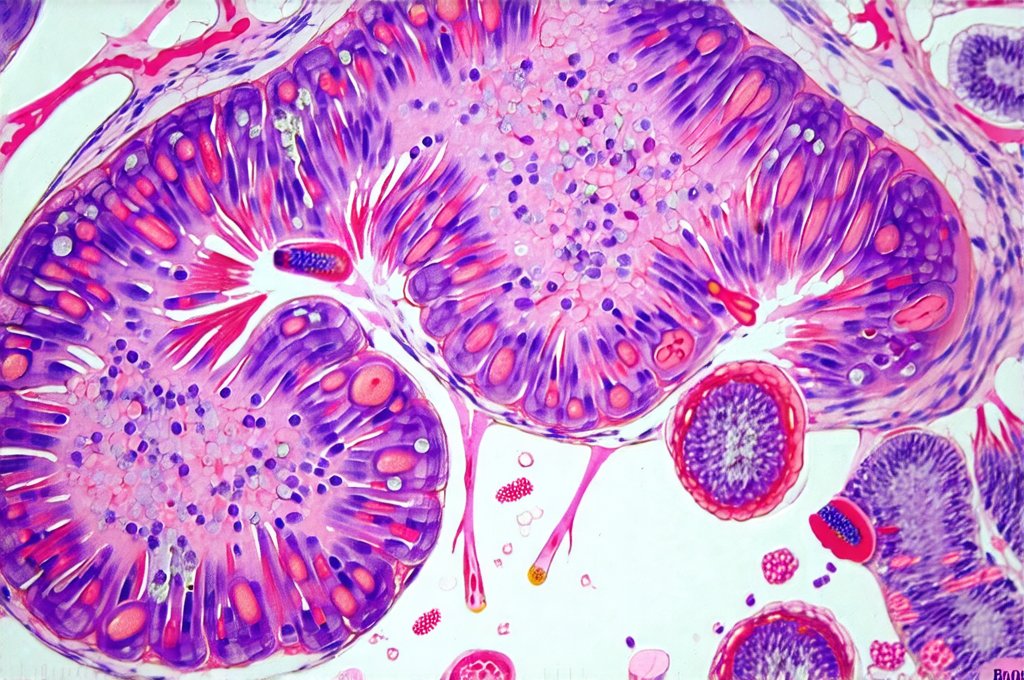
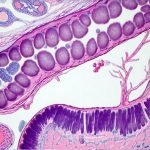
The gut, often referred to as our “second brain,” is far more than just a digestive organ; it’s a central hub for immune function, nutrient absorption, and even mental wellbeing. Increasingly, research highlights the profound connection between gut health and systemic inflammation – meaning inflammation that affects the entire body. When the delicate balance within the gut ecosystem is disrupted—through factors like diet, stress, medication (particularly antibiotics), or infections—it can trigger a cascade of inflammatory responses. Identifying gut inflammation isn’t always straightforward, as symptoms can be vague and mimic other conditions. Traditionally, diagnosis relied heavily on subjective reports of digestive discomfort. However, advancements in laboratory testing now allow us to assess the degree of gut inflammation through specific blood markers, offering objective insights into this often-hidden process.
Understanding these biomarkers is crucial for proactive health management. They provide a window into what’s happening within the intestinal environment, potentially allowing for earlier intervention and personalized strategies to restore balance. It’s important to remember that blood marker results are just one piece of the puzzle; they should always be interpreted in conjunction with a comprehensive clinical evaluation, considering individual symptoms, medical history, and lifestyle factors. This article will explore key blood markers indicative of gut inflammation, outlining their significance and offering a deeper understanding of this complex relationship between our gut and overall health.
Markers of Systemic Inflammation Triggered by Gut Dysfunction
Gut inflammation doesn’t remain isolated to the digestive tract. As intestinal permeability increases – often called “leaky gut” – larger molecules can escape into the bloodstream, triggering an immune response and systemic inflammation. This chronic low-grade inflammation is linked to a wide range of health issues, from autoimmune diseases and allergies to cardiovascular disease and even mental health disorders. Several blood markers reflect this systemic inflammatory state, signaling that something is amiss within the gut environment.
C-reactive protein (CRP) is perhaps the most well-known marker of general inflammation in the body, and its elevation can certainly indicate gut-driven inflammation. However, CRP isn’t specific to the gut; it rises with any inflammatory process. Therefore, while a high CRP level signals inflammation, further investigation is needed to pinpoint the source. More specialized markers offer greater clarity. Fecal calprotectin is often used as a marker for intestinal inflammation and can correlate with increased levels of systemic inflammatory markers like CRP. Serum amyloid A (SAA) is another acute-phase protein that rises rapidly during inflammation, offering similar insights to CRP but sometimes being more sensitive in certain conditions. Elevated SAA alongside gut symptoms warrants further exploration.
Beyond these general inflammatory markers, assessing the immune system’s response provides valuable information. Immunoglobulin G (IgG), IgA and IgM are antibodies produced by the body to fight off pathogens. Elevated levels of specific IgG subtypes can indicate reactivity to food proteins or microbial components that have crossed the intestinal barrier due to increased permeability. While IgG testing for food sensitivities is controversial, it can be a piece of the puzzle when combined with other clinical findings. Similarly, elevated IgA suggests an overactive immune response in the gut, potentially indicating chronic inflammation and dysbiosis (an imbalance in gut bacteria). Considering how diet impacts the gut, exploring strategic meal layers can be a helpful step.
Assessing Intestinal Permeability
Intestinal permeability, often referred to as “leaky gut,” plays a central role in triggering systemic inflammation from gut dysfunction. When the tight junctions between intestinal cells become compromised, larger undigested food particles, toxins, and microbes can enter the bloodstream, activating the immune system. Several blood markers can help assess this permeability, although direct testing methods like lactulose/mannitol breath tests are more commonly used.
- Zonulin: This protein regulates the permeability of tight junctions in the intestinal wall. Elevated zonulin levels indicate a breakdown in gut barrier function, allowing for increased passage of substances into the bloodstream. While still an emerging area of research, zonulin is gaining recognition as a key marker of intestinal permeability.
- Lipopolysaccharide (LPS): LPS is a component of the outer membrane of gram-negative bacteria found in the gut. When the gut barrier is compromised, LPS can enter circulation, triggering a strong immune response and systemic inflammation. Measuring LPS levels or antibodies against it can provide insight into gut leakiness.
- Anti-vinculin antibody: Vinculin is an important protein involved in maintaining the integrity of tight junctions. Antibodies against vinculin suggest that the body is attacking its own intestinal barrier, leading to increased permeability.
It’s crucial to understand that these markers don’t definitively diagnose “leaky gut,” but rather provide evidence of compromised gut barrier function. Addressing factors contributing to increased permeability – such as dietary changes, stress management, and targeted supplementation – can help restore gut health and reduce systemic inflammation. Prioritizing hydration-timed meals is also a key component of gut health.
The Role of Gut Microbiota in Inflammation
The composition of our gut microbiota profoundly influences the degree of inflammation within the gut and throughout the body. A diverse and balanced microbiome contributes to a healthy immune system and reduces inflammatory responses. Conversely, an imbalanced microbiome (dysbiosis), characterized by an overgrowth of harmful bacteria and a lack of beneficial ones, can exacerbate inflammation.
- Fecal markers: While not blood markers directly, analyzing fecal samples provides valuable insights into the gut microbiota composition. Tests like 16S rRNA gene sequencing can identify the types and abundance of bacteria present in the gut, revealing imbalances that contribute to inflammation.
- Short-Chain Fatty Acids (SCFAs): SCFAs, such as butyrate, propionate, and acetate, are produced by beneficial bacteria during the fermentation of dietary fiber. They have anti-inflammatory properties and play a vital role in maintaining gut health. Low levels of SCFAs can indicate dysbiosis and increased inflammation. Although typically measured in stool samples, some research is exploring SCFA measurement in blood.
- Microbial metabolites: The gut microbiome produces various metabolites that impact systemic health. Measuring certain microbial metabolites, such as trimethylamine N-oxide (TMAO), which is linked to cardiovascular disease, can provide insights into the functional activity of the gut microbiota and its influence on inflammation.
Restoring a healthy gut microbiome through dietary changes (increasing fiber intake), probiotic supplementation, and lifestyle modifications can significantly reduce gut inflammation and improve overall health. Food rituals can also help foster a healthy relationship with food and digestion.
Beyond Traditional Markers: Emerging Tests
Research continues to identify novel blood markers that offer more nuanced insights into gut inflammation and dysfunction. These emerging tests are promising but often require further validation before widespread clinical use.
- Myeloperoxidase (MPO): MPO is an enzyme released by neutrophils, a type of white blood cell involved in inflammatory responses. Elevated MPO levels can indicate ongoing inflammation within the gut, even when traditional markers like CRP are normal.
- Lipid mediators: Specialized pro-resolving mediators (SPMs) are molecules that actively resolve inflammation and promote tissue healing. Measuring SPMs can help assess the body’s ability to dampen down inflammatory responses in the gut.
- Cytokine profiles: Cytokines are signaling molecules involved in immune communication. Analyzing specific cytokine levels – such as interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin 1β (IL-1β) – can provide a detailed picture of the inflammatory processes occurring within the gut.
Ultimately, understanding blood markers related to gut inflammation is about more than just numbers on a lab report. It’s about gaining a deeper understanding of the intricate connection between our gut health and overall wellbeing. By utilizing these tools alongside comprehensive clinical assessments, we can develop personalized strategies to restore balance, reduce inflammation, and promote long-term health. Incorporating thermal recipes into your diet can further support gut function, as well as focusing on meal pacing frameworks to optimize digestion. And don’t underestimate the power of midweek dishes designed for gut reset and relief.