Autoimmune diseases are characterized by the immune system mistakenly attacking the body’s own tissues, leading to chronic inflammation and a wide range of symptoms. While many associate autoimmune conditions with joint pain, skin rashes, or fatigue, the digestive system is frequently—and profoundly—affected. In fact, gastrointestinal (GI) involvement isn’t merely a consequence of systemic autoimmunity; it’s often an integral part of disease expression and can even precede other more recognizable symptoms. Understanding the interplay between autoimmune flares and gut health is therefore crucial for effective diagnosis and management.
The complex relationship stems from several factors. The gut microbiome, with its trillions of bacteria, plays a vital role in immune regulation. Disruption of this delicate ecosystem—known as dysbiosis—can trigger inflammation and contribute to autoimmunity. Furthermore, the intestinal barrier, which separates the gut contents from the bloodstream, can become “leaky” in autoimmune conditions (often referred to as ‘leaky gut’), allowing undigested food particles and bacterial toxins to enter circulation, further fueling immune responses. This intricate connection necessitates a sophisticated approach to diagnostics during flares, extending beyond traditional blood tests for inflammatory markers. Identifying digestive involvement is key to tailoring treatment strategies and improving patient outcomes. Considering diagnostics often used in hormonal conditions can be beneficial when assessing these patients.
Diagnostic Approaches Focusing on Gut Involvement
The diagnostic landscape for autoimmune-related GI issues has evolved considerably in recent years. Historically, diagnosis relied heavily on symptom assessment and broad screening tests. Now, a more targeted approach utilizing advanced stool analysis, endoscopic procedures, and specialized blood markers is becoming increasingly common. It’s important to remember that no single test definitively proves an autoimmune connection; rather, clinicians piece together information from multiple sources to create a comprehensive picture. This often requires collaboration between rheumatologists, gastroenterologists, and functional medicine practitioners. A core principle in these investigations is recognizing the variability of symptoms – what affects one person with an autoimmune condition may not be present in another, even with the same diagnosis. Often, digestive testing used in post-antibiotic care can reveal underlying issues contributing to gut dysregulation.
The cornerstone of initial evaluation remains a thorough patient history focusing on GI symptoms like abdominal pain, bloating, diarrhea, constipation, nausea, and changes in bowel habits. These are then coupled with blood tests to assess general inflammation (e.g., C-reactive protein, erythrocyte sedimentation rate) and rule out other potential causes. However, the true power lies in delving deeper into gut-specific diagnostics. Stool testing, for instance, can identify dysbiosis, opportunistic pathogens, markers of inflammation (like calprotectin), and impaired digestion/absorption. Endoscopic procedures like colonoscopy and upper endoscopy allow for direct visualization of the GI tract, biopsy sampling to assess tissue damage, and identification of specific inflammatory patterns. Utilizing digestive panels used in fatigue assessment can help pinpoint contributing factors.
The limitations of traditional diagnostics necessitate a move towards more nuanced testing modalities. For example, breath tests can identify Small Intestinal Bacterial Overgrowth (SIBO), a common condition in autoimmune patients characterized by excessive bacteria in the small intestine, contributing to malabsorption and inflammation. Furthermore, emerging technologies like gut permeability assessments – while still under investigation – offer potential insights into intestinal barrier function. Ultimately, effective diagnosis hinges on recognizing that digestive symptoms are not simply ‘side effects’ of autoimmunity but may be central to disease pathogenesis. It is often helpful to perform digestive diagnostics used before starting serious medication for a more comprehensive understanding.
Biomarkers Beyond Traditional Inflammation
While C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are useful indicators of systemic inflammation, they often fail to capture the nuances of gut-specific inflammation in autoimmune flares. This is where more specialized biomarkers come into play. These markers can help differentiate between inflammatory bowel disease (IBD)-like symptoms caused by autoimmunity versus true IBD, and identify specific mechanisms driving GI dysfunction. It’s crucial to understand that these aren’t always readily available or covered by insurance; however, they represent a significant advancement in diagnostic capabilities.
Fecal calprotectin, as mentioned previously, is a marker of neutrophil activation in the gut, indicating inflammation. Elevated levels suggest intestinal inflammation regardless of its cause but can help assess disease activity and response to treatment. Stool tests analyzing secretory IgA (sIgA) – an antibody crucial for immune defense in the gut – can reveal impaired mucosal immunity. Low sIgA levels are often seen in autoimmune conditions and may indicate a compromised gut barrier. Another increasingly utilized marker is zonulin, a protein that regulates intestinal permeability. Elevated zonulin levels suggest increased “leakiness” of the gut, potentially contributing to systemic inflammation.
Furthermore, assessing antibody reactivity to food proteins and microbial antigens can provide valuable insights. While food sensitivities don’t necessarily cause autoimmunity, they can exacerbate symptoms and contribute to intestinal inflammation during flares. Testing for antibodies against specific bacterial species (e.g., lipopolysaccharide – LPS) may indicate increased gut permeability and immune reactivity to gut microbes. These biomarkers offer a more targeted approach to understanding the complex interplay between autoimmune disease, gut health, and inflammation. The need for investigations used in pediatric digestive complaints can often be overlooked in adult populations as well.
The Role of Stool Analysis
Stool analysis has transformed from a basic test for detecting parasites to a comprehensive evaluation of gut health. Modern stool tests can now provide detailed information about the microbiome composition, inflammatory markers, digestive function, and potential pathogens. This makes it an invaluable tool in diagnosing autoimmune-related GI issues during flares. A crucial aspect is choosing the right test – there’s significant variability among different labs and testing methodologies.
The process typically involves collecting a small stool sample at home using provided instructions, ensuring proper collection techniques to avoid contamination. The sample is then sent to a specialized laboratory for analysis. Results are usually available within a week or two, depending on the lab. Interpretation requires expertise, as understanding microbiome data can be complex. Key parameters assessed include: – Microbial diversity (a measure of gut health) – Relative abundance of specific bacterial species (identifying imbalances and potential pathogens) – Markers of inflammation (e.g., calprotectin, lactoferrin) – Digestive enzyme function (assessing malabsorption risks) – Short-chain fatty acid (SCFA) production (indicators of microbial fermentation and gut health).
It’s important to note that stool analysis provides a snapshot in time – the microbiome is dynamic and can change rapidly. Therefore, repeat testing may be necessary to assess treatment effectiveness or track disease progression. Also, results should always be interpreted within the context of the patient’s clinical presentation and other diagnostic findings. Stool analysis isn’t merely about identifying ‘bad bacteria’; it’s about understanding the overall ecosystem of the gut and how it relates to autoimmune disease activity. Utilizing digestive assessments used in chronic skin conditions can reveal valuable insights for treatment planning.
Endoscopic Procedures & Biopsies
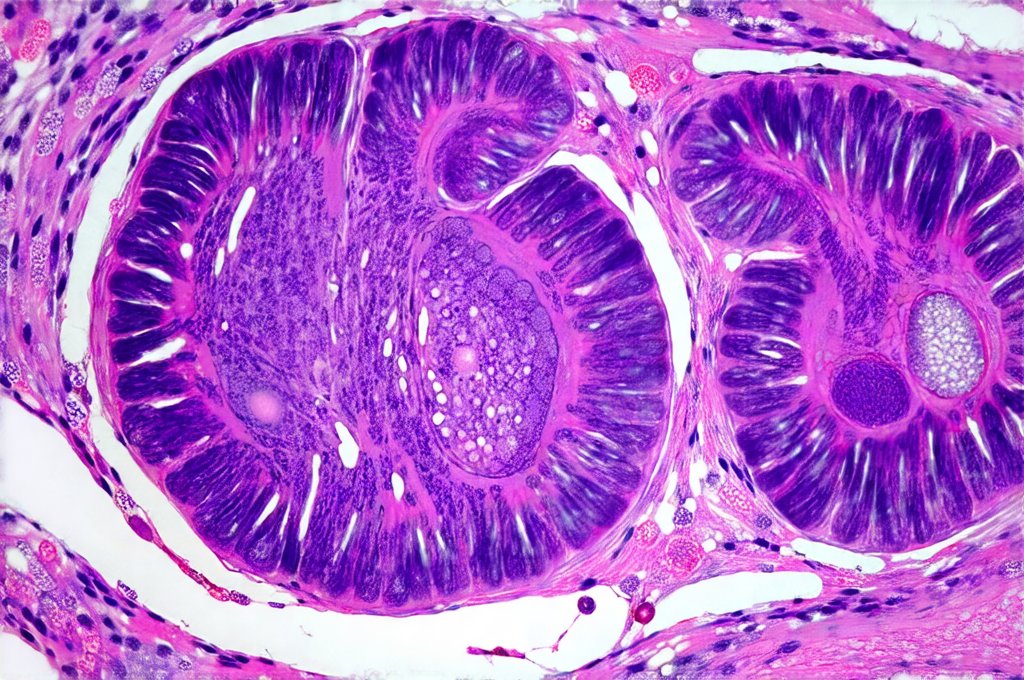
When stool analysis and blood tests suggest significant GI involvement, endoscopic procedures become essential for direct visualization and tissue sampling. Colonoscopy involves inserting a flexible tube with a camera into the rectum to examine the entire colon, while upper endoscopy examines the esophagus, stomach, and duodenum. These procedures allow clinicians to identify inflammation, ulcers, polyps, or other abnormalities in the digestive tract.
During endoscopy, biopsies – small tissue samples – are taken from areas of concern for microscopic examination by pathologists. This is crucial for differentiating between various causes of intestinal inflammation. For example, biopsies can help distinguish autoimmune-related colitis from Crohn’s disease or ulcerative colitis. They also reveal specific histological features indicating the type and severity of inflammation. The procedure itself requires bowel preparation beforehand to ensure clear visualization, and patients are typically sedated for comfort.
It’s worth noting that endoscopic findings in autoimmune conditions may be subtle compared to classic IBD presentations. Inflammation might be patchy or less severe, making accurate diagnosis challenging. However, biopsies provide valuable objective evidence supporting the presence of GI involvement. Endoscopic procedures aren’t simply diagnostic tools; they are essential for guiding treatment decisions and monitoring disease activity.
Emerging Diagnostic Technologies
The field of autoimmune diagnostics is constantly evolving, with new technologies emerging to improve our understanding of gut health and its role in flares. One promising area is metabolomics, which analyzes the small molecules produced by metabolic processes in the body. Analyzing fecal metabolomes can reveal unique metabolic signatures associated with different autoimmune conditions and their impact on GI function.
Another developing technology is advanced microbiome sequencing, going beyond 16S rRNA gene sequencing to whole-genome shotgun metagenomic sequencing, providing a more detailed understanding of microbial composition and functional capabilities. This allows for identification of specific genes involved in inflammation or gut barrier disruption. Finally, research into gut permeability assessments continues, with non-invasive methods like lactulose/mannitol breath tests gaining traction as potential indicators of intestinal leakiness.
These technologies are still under investigation and not widely available; however, they hold tremendous promise for the future of autoimmune diagnostics. They represent a shift towards personalized medicine, tailoring diagnostic and treatment strategies based on an individual’s unique gut microbiome and metabolic profile. The ongoing development of these tools underscores the growing recognition of the crucial role of the digestive system in autoimmune disease. Recognizing top overlooked tools in everyday GI diagnostics is critical for comprehensive patient care.