The esophagus, often overlooked in discussions of digestive health, is a remarkably dynamic organ. It’s not simply a conduit for food; it’s a complex muscular tube subject to constant stress from acidic contents, mechanical stretching during swallowing, and potential injury from foreign bodies or chemical irritation. Consequently, the esophageal lining requires robust mechanisms for repair and regeneration when damage occurs. While many cellular processes contribute to healing, collagen plays an absolutely pivotal role – acting as both a structural component of the tissue itself and a key player in the wound-healing cascade. Understanding this role is crucial not only for appreciating esophageal health but also for developing effective strategies for managing conditions like esophagitis, Barrett’s esophagus, and esophageal strictures. Maintaining adequate hydration can support overall healing processes within the digestive system.
The process of esophageal healing isn’t just about patching up holes; it’s about restoring functional integrity. This means rebuilding the layered structure of the esophageal wall – from the innermost mucosal layer to the outer adventitia – with a focus on maintaining barrier function and preventing further damage. Collagen, in its various forms, is integral to this process. It provides the scaffolding for cellular attachment, guides tissue organization during repair, and influences the inflammatory response that dictates how quickly and effectively healing occurs. Disruptions in collagen synthesis or degradation can significantly impair esophageal regeneration, leading to chronic inflammation and potentially increasing the risk of complications. The connection between gut health and overall inflammatory response is also important to consider here.
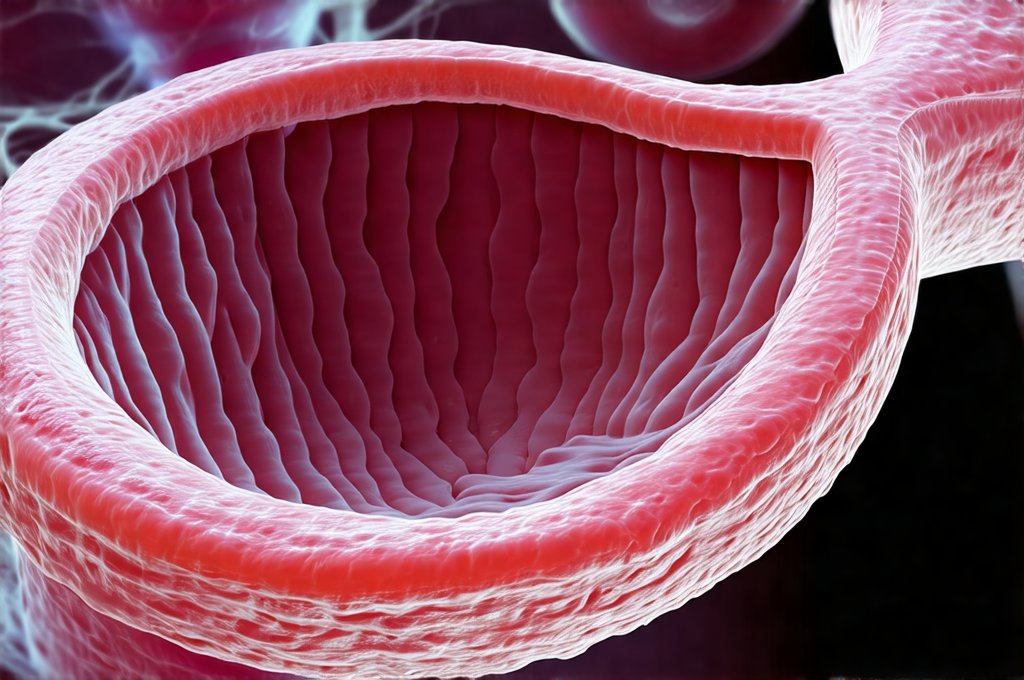
Collagen Structure & Types in Esophageal Tissue
Collagen isn’t a single entity; it’s actually a family of proteins with diverse structures and functions. In the esophagus specifically, several types are prominent. Type I collagen is by far the most abundant, forming the bulk of the fibrous connective tissue within all layers of the esophageal wall – providing tensile strength and resisting stretching forces. Type III collagen, often found alongside type I, contributes to the early stages of wound healing and provides a more pliable framework for initial repair. Type IV collagen is crucial in the basement membrane, supporting epithelial cell attachment and maintaining barrier integrity. Finally, smaller amounts of other types like V and VI contribute to tissue organization and fibrillogenesis – the process of forming collagen fibers.
The unique arrangement of these collagen types creates a resilient yet adaptable esophageal wall. Think of it as an intricately woven fabric; type I provides the strong warp threads, type III offers early support and flexibility, and type IV anchors everything together at the cellular level. This layered structure is essential for withstanding the mechanical stresses of swallowing and protecting underlying tissues from acid exposure. When damage occurs, the body aims to recreate this specific arrangement during healing, relying heavily on fibroblasts – the cells responsible for collagen synthesis – to lay down new fibers in a coordinated manner. Understanding gut microbiome influence can also impact tissue regeneration.
Collagen molecules themselves are formed through a complex process involving procollagen synthesis within fibroblasts, followed by post-translational modifications and extracellular assembly into fibrils and ultimately, mature collagen fibers. This process is tightly regulated by growth factors, cytokines, and enzymes like matrix metalloproteinases (MMPs), which balance collagen synthesis and degradation. An imbalance between these processes – too much breakdown or insufficient production – can lead to impaired healing and structural abnormalities within the esophageal wall.
Collagen’s Role in Inflammation & Wound Healing Phases
Esophageal injury triggers a cascade of inflammatory events, initiating the wound-healing process. Initially, neutrophils arrive at the site of damage to clear debris and prevent infection. This is followed by macrophages which further clean up damaged tissue and release growth factors that stimulate fibroblast activity. Collagen synthesis doesn’t happen immediately; it’s part of the proliferative phase, occurring after initial inflammation subsides. However, collagen deposition isn’t simply a late-stage event; it interacts with inflammatory cells throughout the process.
- Collagen fragments released during tissue damage can actually activate immune cells, amplifying the inflammatory response. This seemingly paradoxical effect highlights the complex interplay between collagen and immunity.
- Growth factors like TGF-β (transforming growth factor beta) are pivotal in stimulating fibroblast proliferation and collagen production. They also play a role in modulating inflammation, helping to transition from acute to chronic phases of healing.
The final phase of wound healing – remodeling – involves reorganization of the newly deposited collagen fibers, increasing their strength and aligning them along lines of stress. This process can take months or even years, and it’s often where complications arise if collagen synthesis is disrupted or if excessive scarring occurs, leading to stricture formation. Maintaining a balanced inflammatory response and supporting optimal fibroblast function are therefore crucial for achieving complete and functional esophageal healing. The role of nutritionists in managing inflammation is increasingly recognized.
Collagen & Esophageal Strictures: A Complication of Healing
Esophageal strictures – narrowings of the esophagus that can make swallowing difficult – are a common complication of chronic inflammation or injury. While many factors contribute to their development, excessive collagen deposition during the remodeling phase is often a key driver. When tissue repair occurs in an environment of persistent inflammation (as seen in reflux esophagitis for instance), fibroblasts can become overly stimulated, leading to excessive and disorganized collagen production.
This results in dense scar tissue that progressively narrows the esophageal lumen. The problem isn’t necessarily a lack of collagen, but rather its misregulation. Treatments for esophageal strictures often focus on mechanically dilating the narrowed area using endoscopic balloon dilation – temporarily stretching the esophagus. However, without addressing the underlying cause of inflammation and promoting more balanced collagen remodeling, strictures frequently recur.
- Endoscopic therapies aim to reduce inflammation and promote healthy tissue regeneration, but they don’t directly alter collagen deposition.
- Research is exploring potential therapeutic strategies to modulate collagen synthesis and degradation – for example, using MMP inhibitors or growth factor antagonists – to prevent excessive scarring and improve long-term outcomes. However, these approaches are still under investigation.
The Future of Collagen-Based Therapies in Esophageal Healing
Current research is actively investigating ways to harness the power of collagen to enhance esophageal healing. One promising area is collagen-based biomaterials. These materials can be used as scaffolds to support tissue regeneration, delivering growth factors directly to the site of injury and promoting organized collagen deposition. For example, collagen hydrogels containing cells or bioactive molecules are being explored as potential treatments for Barrett’s esophagus – a precancerous condition where the esophageal lining is replaced by intestinal-like cells.
Another avenue of research involves modulating collagen synthesis through targeted therapies. This could involve using small molecule inhibitors to block excessive fibroblast activation, or delivering genes that promote balanced collagen production. Furthermore, understanding the specific collagen types and their arrangement within healthy versus diseased esophageal tissue can help identify novel therapeutic targets.
- Personalized medicine approaches, tailoring treatments based on individual patient characteristics and the specific type of esophageal injury, may become more common as our understanding of collagen’s role in healing evolves. Exploring diet can also play a significant role in supporting overall health during treatment.
- Non-invasive imaging techniques to assess collagen content and organization within the esophageal wall are also being developed, offering a way to monitor treatment response and predict outcomes.
Ultimately, unlocking the full potential of collagen-based therapies requires a deeper understanding of the complex interplay between collagen, inflammation, cellular signaling, and tissue remodeling in the esophagus. By focusing on restoring not just structural integrity but also functional barrier properties, we can pave the way for more effective and lasting solutions for esophageal diseases. The support from caregivers is also essential throughout the healing process. Finally, understanding liver regulation can help optimize overall health and recovery.